.jpg/jcr:content/SteamOneLogo%20(1).jpg)
Latest news
28.10.2025
- Protocol Amendment Version 2.1 now active in Germany and Switzerland.
- Home uroflowmetry (Emano-Flow) is now ready for use in Germany and Switzerland.
- The first 100 patients have been operated on.
SteamOne
Study information page of SteamOne - Prospective database for recum water vapor therapy of the prostate
This platform provides information for patients who are treated with Rezum steam therapy in a participating study center as part of SteamOne, as well as for professionals from participating study centers.
Director of Studies

Study coordinator

Contact us
Phone +41 61 328 56 59
E-mail: SteamOne.Urologie@usb.ch
Latest news
28.10.2025
- Protocol amendment version 2.1: Includes the conversion of home uroflowmetry to the new AI-based system from Emano-Flow, as well as the integration of a medication diary for patients to support the documentation of BPH medication, painkillers and blood-thinning medication. The switch to Emano-Flow has become necessary due to the discontinuation of product support from Kesem-Health for the iUFlow homeuroflowmetry device. Now active in Germany and Switzerland.
- Emano-Flow: Now available for study patients in Germany and Switzerland as part of the SteamOne study.
- Abstract submission for the EAU (European Asociation of Urology) Congress 2026 in London.
Patient recruitment


Information for patients
Aim of the study
Benign prostate enlargement is one of the most common diseases in ageing men. However, the glandular tissue of the prostate in the transition zone can begin to grow from the age of 30. Statistically speaking, half of all men between the ages of 50 and 60 already have benign prostatic hyperplasia (BPH) (1). The excess tissue can lead to a narrowing of the prostatic urethra, which is referred to as benign prostatic obstruction (BPO), which can lead to an increase in bladder outlet resistance. BPO can lead to stressful symptoms of the lower urinary tract, so-called "lower urinary tract symptoms" (LUTS).
The incidence of LUTS due to BPO is 3/100,000 men per year in the 45-49 age group and rises to a maximum of 38/100,000 men per year in the 75-79 age group. For a symptom-free man aged 46, the risk of developing BPO-related LUTS in the next 30 years is therefore 45% (2).
There is a demonstrable need for patient-centered treatment decisions that focus on the patient's needs. Accordingly, a large number of minimally invasive treatment techniques have been developed in recent years (3).
Rezum water vapor therapy of the prostate is understandably arousing the interest of treating physicians and men suffering from BPO-induced LUTS, as Rezum has been shown to be able to significantly improve urination (4,5) as well as protect sexual function (6,7). In addition, Rezum can be performed under local anesthesia and without general or spinal anesthesia, which means that older or multimorbid patients can also be treated with Rezum. However, further studies, such as SteamOne, are needed to validate the promising data from the aforementioned pivotal studies, which will hopefully lead to evidence- and guideline-based recommendations for Rezum in the future in order to better advise and treat patients.
The aim of the SteamOne study is to further improve the data available on Rezum treatment. Around 1000 patients will be observed and data collected over a period of 5 years as part of routine treatment with Rezum.
The focus of data collection includes
- Benefits of the Rezum treatment
- Satisfaction with the Rezum treatment
- Influence of Rezum on quality of life
- Influence of Rezum on sexual function
- Side effects and complications of Rezum
- Recovery from Rezum treatment
- Patient expectations of the Rezum treatment
- Change in urodynamic values (values from bladder function measurements)
The aim is to take a further step towards creating treatment recommendations for specialists. At the same time, the counseling options for patients should be improved in the course of SteamOne. Last but not least, the study will make an important contribution to research.
Participation requirements
Inclusion criteria:
- Basically all patients who are treated for BPO/LUTS in the participating study centers
- Age ≥ 18
- The indication for Rezum must be made independently of the study
- The decision to perform Rezum is the responsibility of the patient and the treating medical staff
- Operated or supervised by certified urologist/urologist
- Subgroups of particular interest are e.g. catheter-dependent patients, patients on oral anticoagulation, patients with preoperative urodynamic pressure-flow examination (not older than 6 months) or patients with a prostate of more than 80 ml
Exclusion criteria:
- Lack of informed consent
- Inability to answer questionnaires independently in German (e.g. dementia, mental disability)
- The patient does not have a personal e-mail address and cannot complete the survey using a relative's e-mail address and is not willing to complete the survey on the tablet in the clinic
- Known or suspected neurogenic bladder dysfunction (e.g. Parkinson's disease, multiple sclerosis or other neurogenic diseases with possible bladder dysfunction)
- History of bladder tumor in the last two years (incl. CIS) or bladder tumor at the time of recurrence treatment (incl. CIS)
- Previous prostate procedures, except prostate biopsies, if these were performed more than 4 weeks prior to the recurrence procedure
- Previous bladder neck surgery
- Bladder neck stenosis requiring treatment at the time of recum treatment
- Planned combination of a simultaneous Rezum treatment with another urological* or medical procedure
* Combination with a planned transurethral procedure is also an exclusion criterion, except in the case of bladder stone removal
Expenditure
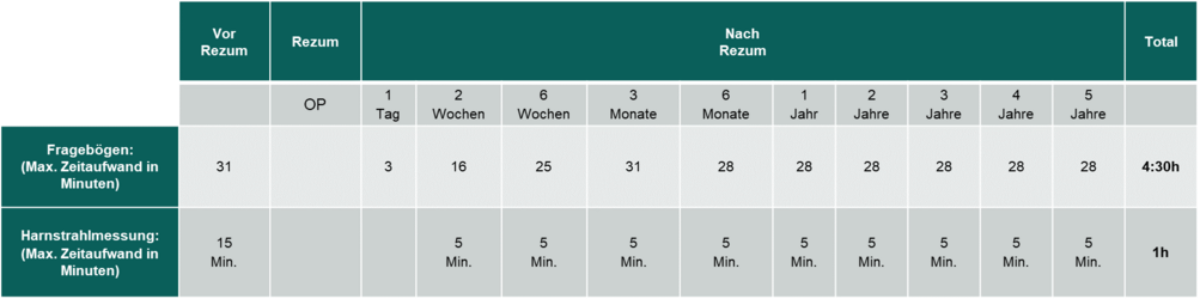
As a study patient, you will be asked to answer various questionnaires at certain survey times. These will be sent to you by email link via the study database and can be completed conveniently at home. Study patients will also receive a urinary flow meter (smartphone app with AI (artificial intelligence) for urinary flow measurement at home free of charge. The data collected in this way is automatically transferred to the study database of the University Hospital Basel via app and smartphone. The time required and the survey and measurement times can be found in the table below.
Risks
No health risks are to be expected.
Benefit
- All data will be treated confidentially.
- Participation in the study is free of charge for patients.
- Study patients receive their own urine flow meter (KI app for smartphone) to use at home.
- Some of the questionnaires are also used for follow-up examinations. These can be completed easily and efficiently at home, which means less time in the clinic for study patients.
- Urinary stream measurements do not have to be carried out "on command" in the clinic, but can be carried out at home at the appropriate time, which should increase the quality of the measurements.
- The data is automatically and easily transmitted to the study database via an app or e-mail link.
- Last but not least, your own state of health is documented, which is valuable both for you and for the clinic treating you.
Participating study centers
SteamOne is a multicenter, trinational study. The University Hospital Basel is the coordinating center.
The following centers are participating in the study.
Switzerland
- University Hospital Basel (USB)
- Aarau Cantonal Hospital (KSA)
- Hirslandenklinik St. Anna, Lucerne (SAL)
- St. Gallen Cantonal Hospital (KSG)
- Lucerne Cantonal Hospital (LUK)
- Inselspital Bern (INB)
- Uroviva Rothrist (UVR)
- University Hospital Zurich (USZ)
Austria
- University Hospital Graz (UKG)
- Confraternity of Vienna (COW)
Germany
- Maria-Hilf Krefeld / Alexianer (MHK)
- Nuremberg North Hospital (KNB)
- Reinbek Hospital (KRE)
- Urodock - Urological Group Practice, Kiel (UDK)
- University Hospital Frankfurt (KGU)
- Alexianer St. Hedwig Hospital, Berlin (SHK)
- Asklepios Clinics (Westklinikum) Hamburg (WKH)
Information for specialist staff
Aim of the study
The Rezum procedure is attracting increasing attention from both patients and specialists. Unfortunately, the current data situation is limited. So far, mainly cohort studies with relatively small numbers of cases have been conducted. These studies are often retrospective, and subgroup analyses are lacking. These study limitations mean that there are currently still no recommendations on the Rezum procedure in urological guidelines (for example, the guideline of the European Association of Urology (EAU): Management of Non-neurogenic Male LUTS).
SteamOne is intended to help remedy this deficit.
The focus of data collection includes
- Benefits of the Rezum treatment
- Satisfaction with the Rezum treatment
- Influence of Rezum on quality of life
- Influence of Rezum on sexual function
- Side effects and complications of Rezum
- Recovery from Rezum treatment
- Patient expectations of the Rezum treatment
- Change in urodynamic values (values from bladder function measurements)
The aim is to take a further step towards creating treatment recommendations for specialists. At the same time, the counseling options for patients should be improved in the course of SteamOne. Last but not least, the study will make an important contribution to research.
Participation requirements
Study centers
Clinics qualify as study centers if they have already performed more than 50 Rezum treatments and see potential to recruit 50 patients within 12-18 months.
The Rezum procedure must be performed by a certified urologist who has successfully completed a Rezum training course.
Study population
Inclusion criteria:
- Basically all patients who are treated for BPO/LUTS in the participating study centers
- Age ≥ 18
- The indication for Rezum must be made independently of the study
- The decision to perform Rezum is the responsibility of the patient and the treating medical staff
- Operated or supervised by certified urologist/urologist
- Subgroups of particular interest are e.g. catheter-dependent patients, patients on oral anticoagulation, patients with preoperative urodynamic pressure-flow examination (not older than 6 months) or patients with a prostate of more than 80
Exclusion criteria:
- Lack of informed consent
- Inability to answer questionnaires independently in German (e.g. dementia, mental disability)
- The patient does not have a personal e-mail address and cannot complete the survey using a relative's e-mail address and is not willing to complete the survey on the tablet in the clinic
- Known or suspected neurogenic bladder dysfunction (e.g. Parkinson's disease, multiple sclerosis or other neurogenic diseases with possible bladder dysfunction)
- History of bladder tumor in the last two years (incl. CIS) or bladder tumor at the time of recurrence treatment (incl. CIS)
- Previous prostate procedures, except prostate biopsies, if these were performed more than 4 weeks prior to the recurrence procedure
- Previous bladder neck surgery
- Bladder neck stenosis requiring treatment at the time of recum treatment
- Planned combination of a simultaneous Rezum treatment with another urological* or medical procedure
* Combination with a planned transurethral procedure is also an exclusion criterion, except in the case of bladder stone removal
Study design
SteamOne is designed as a prospective, multicenter observational study. The aim is to recruit 1000 patients in Switzerland, Germany and Austria at a maximum of 25 study centers over a period of 18-24 months as part of routine treatment with Rezum. PROMs (Patient Reported Outcome Measures) and CROMs (Clincal Reported Outcome Measures) will be collected over a follow-up period of 5 years. The web-based platform "REDCap" serves as the survey portal and study database.
Patients receive questionnaires on the various focal points via an uncomplicated email link and complete them electronically themselves. As part of the study, each participating center receives its own licensed version of the REDCap software with an associated local database for entering clinical parameters. The data is automatically transmitted electronically via a secure connection to the main study center at the University Hospital Basel. In addition, each participating patient receives an approved and certified home uroflowmetry device using a smartphone (EmanoFlow). This enables patients to take uroflowmetry measurements from home. Here too, data transmission is electronically encrypted and automated via a smartphone app.
Compensation
Participating study centers receive an expense allowance of 500 euros per patient.
PROMs and patient questionnaires
Participating study centers
SteamOne is a multicenter, trinational study. The University Hospital Basel is the coordinating center.
The following centers are participating in the study.
Switzerland
- University Hospital Basel (USB)
- Aarau Cantonal Hospital (KSA)
- Hirslandenklinik St. Anna, Lucerne (SAL)
- St. Gallen Cantonal Hospital (KSG)
- Lucerne Cantonal Hospital (LUK)
- Inselspital Bern (INB)
- Uroviva Rothrist (UVR)
- University Hospital Zurich (USZ)
Austria
- University Hospital Graz (UKG)
- Confraternity of Vienna (COW)
Germany
- Maria-Hilf Krefeld / Alexianer (MHK)
- Nuremberg North Hospital (KNB)
- Reinbek Hospital (KRE)
- Urodock - Urological Group Practice, Kiel (UDK)
- University Hospital Frankfurt (KGU)
- Alexianer St. Hedwig Hospital, Berlin (SHK)
- Asklepios Clinics (Westklinikum) Hamburg (WKH)
Further information can be found here:
For study participants
For study centers
Documents
Study registration