Neurostatus-UHB AG (Ltd.)
Based in Basel, Switzerland, as part of the University Hospital Basel (UHB), Neurostatus-UHB Ltd. is the leading provider of expertise in the standardized neurological assessment of clinical symptoms in Multiple Sclerosis (MS) and related neuro-immunological diseases, the Neurostatus-EDSS. Further, Neurostatus-UHB develops digital solutions for the capture, quantification and documentation of clinical symptoms and has expertise in software validation.
Neurostatus-UHB Ltd. provides services for randomized controlled trials (RCTs), academic studies and clinical routine. These include:
- Licenses for the paper & pen version of the Neurostatus-EDSS
- Licenses for the digital version of the Neurostatus-EDSS, the Neurostatus-eEDSS. The Neurostatus-eEDSS provides an algorithm-based consistency check and automated feedback on the Neurostatus-EDSS assessments data. Assessments labelled as inconsistent are reviewed by our team of EDSS experts who support the rater (i.e. user) in their resolution. The eEDSS is currently being provided by four external electronic clinical outcome assessment (eCOA) companies under non-exclusive licenses, and through our in-house digital solutions, depending on the studies’ requirements and complexities.
- An online certification process (the Neurostatus-eTest) for Neurostatus-(e)EDSS raters, as an element of quality control for Neurostatus-(e)EDSS data
- Training, both face-to-face and online, and the provision of training and scoring materials
All further information about Neurostatus-UHB can be found on the company website.
Illustration of the Neurostatus-eEDSS eCOA feedback cycle and review platform
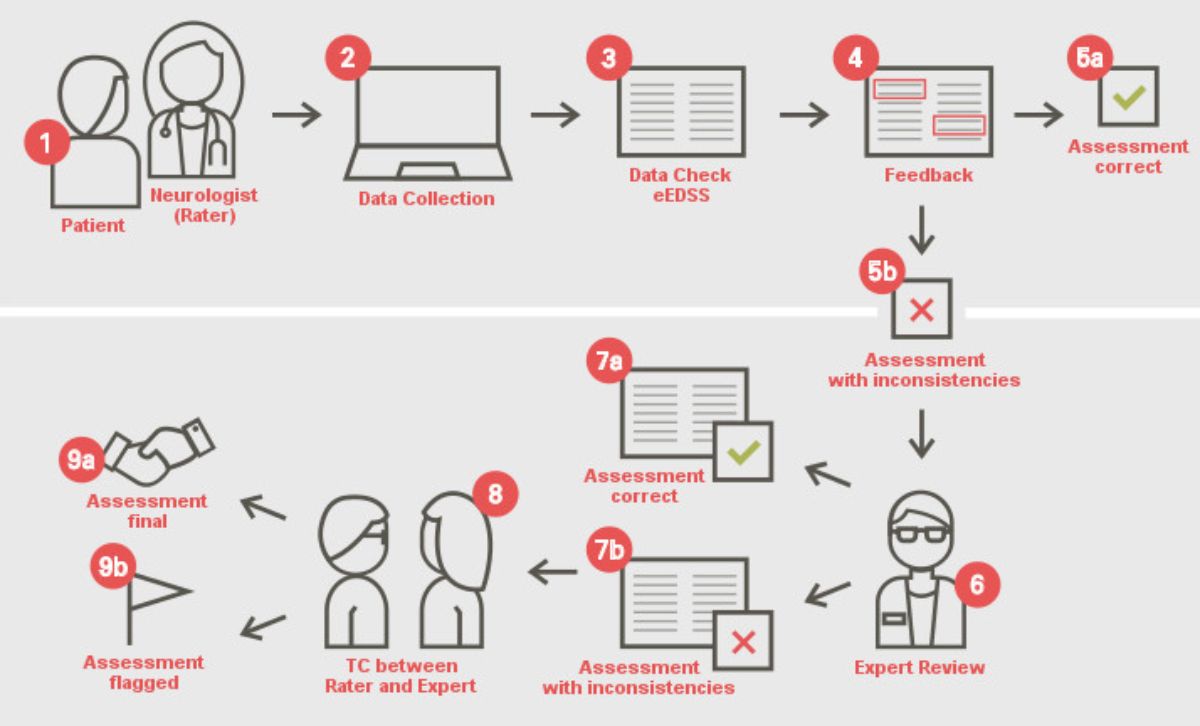
- Numbers 1 to 5a represent the automated real-time feedback;
- Numbers 5b to 9b the expert interaction with on-site raters via eCOA platforms.
Contact for further information
General inquiries:

Scientific or medical inquiries:

PD Dr. Marcus D'Souza
CEO Neurostatus-UHB AG (Ltd.), Senior Physician, Department of Neurology, University Hospital Basel
Tel. +41 61 556 85 54
History and current status (2025)
The Neurostatus-EDSS scale was first introduced in the early 1990s by Professor Ludwig Kappos and his team at the University Hospital Basel, as part of an MS research group.
Since then it has become the “gold-standard” for use during phase III RCTs in MS, including most noteworthy for those trials that led to the approval of the currently used disease modifying treatments (DMTs).
In 2009, led by the current CEO, PD Dr. med. Marcus D’Souza, the Neurostatus-eEDSS was introduced. Along with the COO, Evy Fricker, and their team, they are currently supporting more than 100 active MS and related disorders studies, more than 30 of which are using the digital eEDSS.
In November 2021, the initial research group was incorporated as a limited company wholly owned by the University Hospital Basel.
Current projects
SMARTCARE (NCT05575843)
A Swiss multicenter, randomized, cross-over study with 100 people with MS showed that Non-Neurologist Health Care Professionals (HCPs) are able to perform standardised Neurostatus-(e)EDSS assessments of similar quality and reliability as trained neurologists. A paper presenting results of this first analysis has been just recently published in MSJ. A follow-up trial is in progress.
eCluster
Artificial intelligence (AI) methods were applied on > 13.000 eEDSS assessments from the EXPAND trial, aiming to achieve more granular (sub)gradings in the EDSS scores ³ 4.5 that – in the current version – are dominated by ambulation and do not take into account changes in other functional domains. In this analysis we have found 4 patterns (A to D) within each of the EDSS levels from 4,5 to 6,5. A paper presenting results of this first analysis has been just recently published in MSJ.
Validation
With our deep expertise in regulatory compliance, quality management systems, and validation practices, we aim to support organizations in meeting international standards while ensuring efficient, compliant, and high-quality operations. Our QA and CSV services are designed to cater to the regulated life science industries, ensuring compliance with regulatory frameworks such as FDA 21 CFR Part 11 and 820, ISO 9001, ISO 13485, GxP, and EU MDR. For more information, please contact annalea.gysin@usb.ch.
Domain specific language
Creation of a domain-specific language for use in neurological scales, in order to verify the correctness of data entered according to the rules of set medical scales.
e-Learning
Development of a platform to educate Neurologists and other HCPs on how to perform a standardized neurological examination using video-technology.
